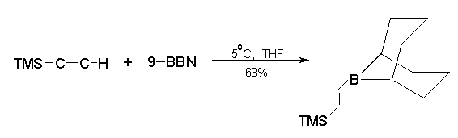
Recent advances in the application of nano-catalysts for Hiyama cross-coupling reactions - RSC Advances (RSC Publishing)
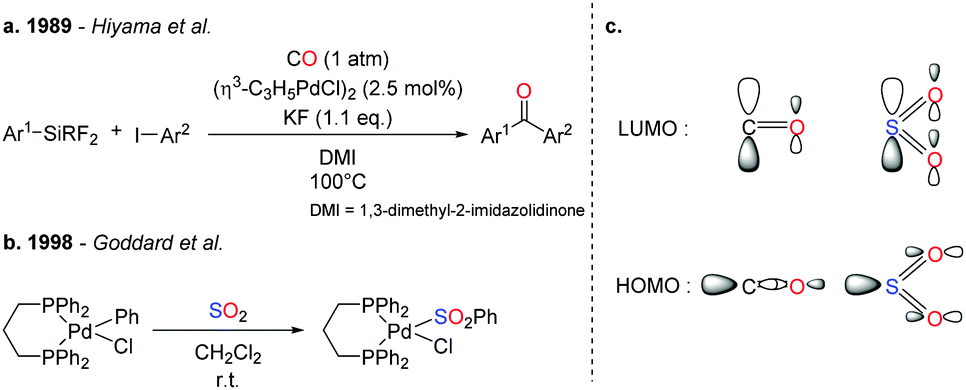
SO 2 conversion to sulfones: development and mechanistic insights of a sulfonylative Hiyama cross-coupling - Chemical Communications (RSC Publishing) DOI:10.1039/C9CC06858A
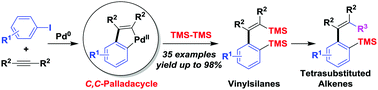
Palladium-catalyzed sequential three-component reactions to access vinylsilanes - Chemical Communications (RSC Publishing)
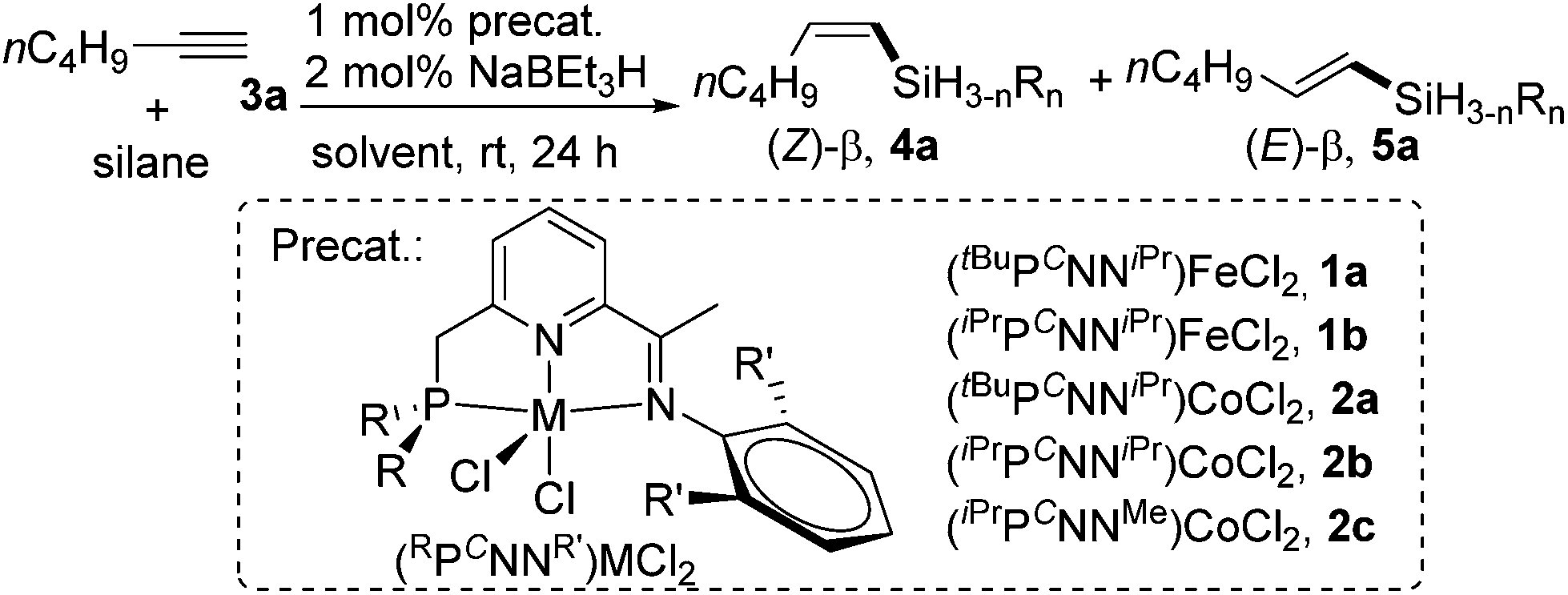
Pincer cobalt complex-catalyzed Z -selective hydrosilylation of terminal alkynes - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/C7QO00250E
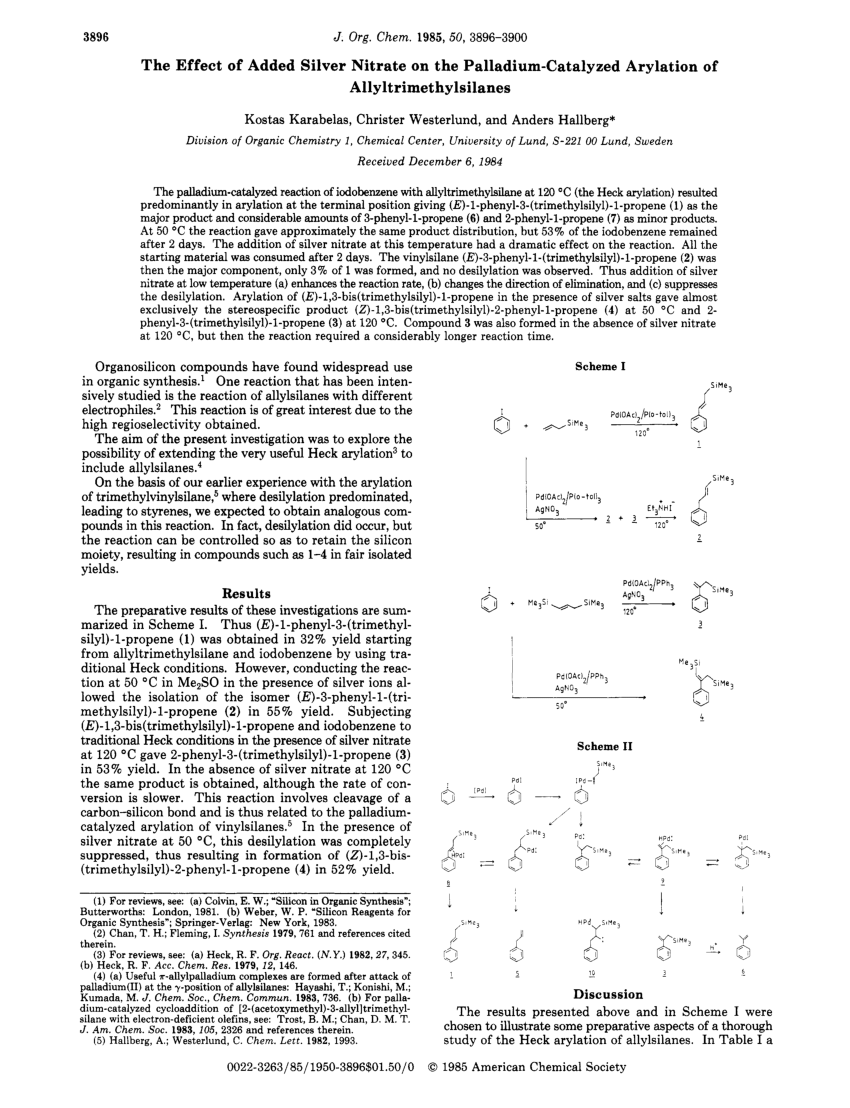
PDF) The effect of added silver nitrate on the palladium-catalyzed arylation of allyltrimethylsilanes
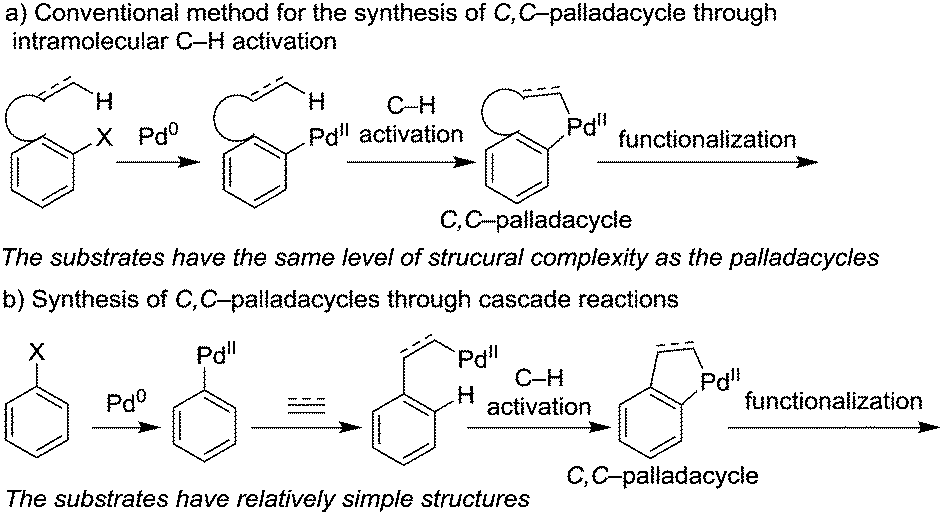
Palladium-catalyzed sequential three-component reactions to access vinylsilanes - Chemical Communications (RSC Publishing) DOI:10.1039/C8CC05254A

Stereoselective Synthesis of Cis- and Trans-Tetrasubstituted Vinyl Silanes Using a Silyl-Heck Strategy and Hiyama Conditions for Their Cross-Coupling. - J. Am. Chem. Soc. - X-MOL

Solvent interception, heterocyclization and desilylation upon NBS-induced sulfamidation of trimethyl(vinyl)silane. - Org. Biomol. Chem. - X-MOL

Inorganics | Free Full-Text | Non-Selective Dimerization of Vinyl Silanes by the Putative (Phenanthroline)PdMe Cation to 1,4-Bis(trialkoxysilyl)butenes | HTML

Table 1 from Palladium-Catalyzed Ortho-Silylation of Aryl Iodides with Concomitant Arylsilylation of Oxanorbornadiene: Accessing Functionalized ( Z)-β-Substituted Vinylsilanes and Their Analogues. | Semantic Scholar
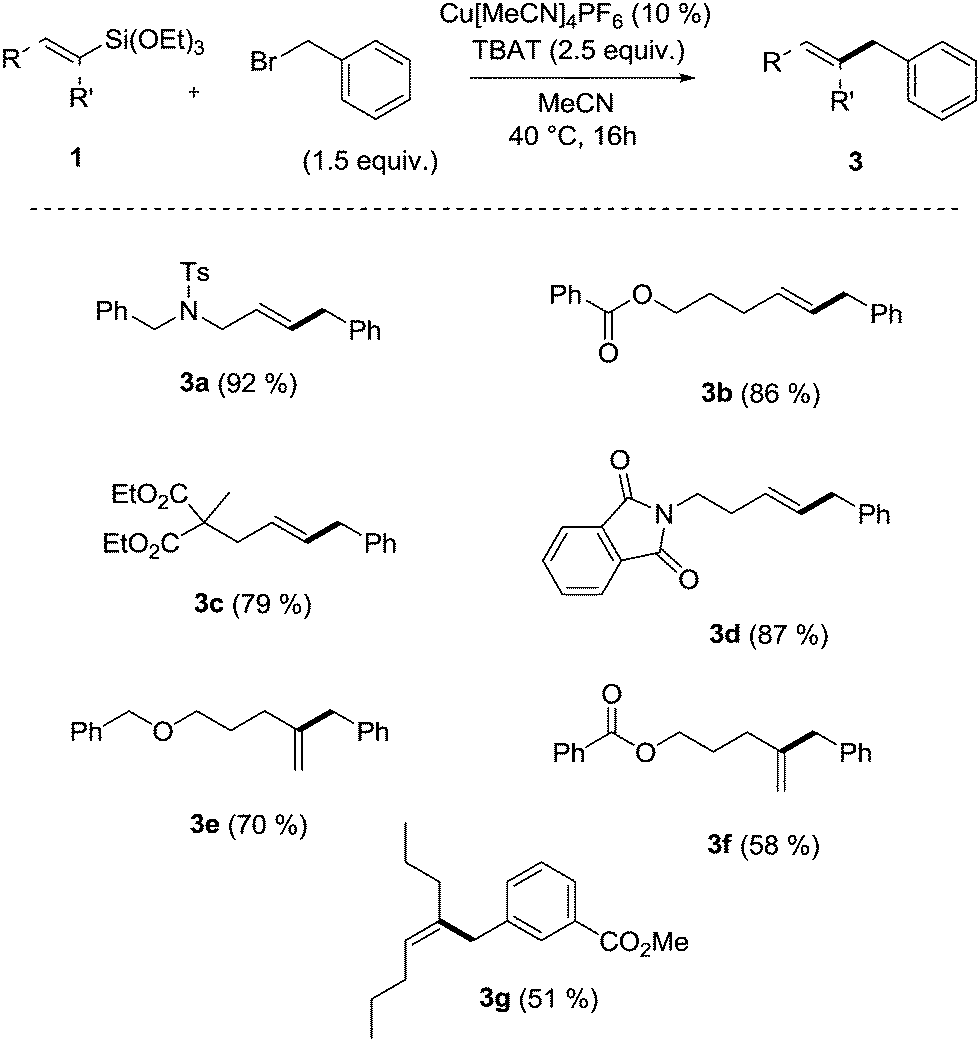
Copper-catalyzed Hiyama cross-coupling using vinylsilanes and benzylic electrophiles - Chemical Communications (RSC Publishing) DOI:10.1039/C4CC02923B
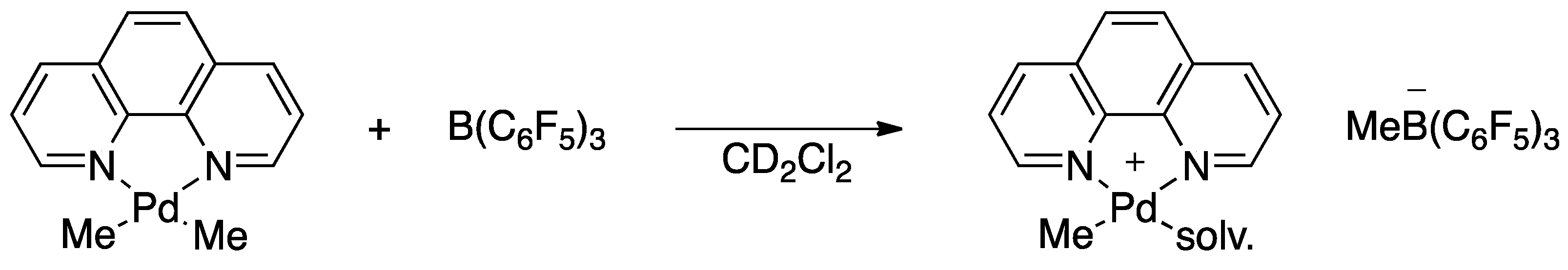
Inorganics | Free Full-Text | Non-Selective Dimerization of Vinyl Silanes by the Putative (Phenanthroline)PdMe Cation to 1,4-Bis(trialkoxysilyl)butenes | HTML
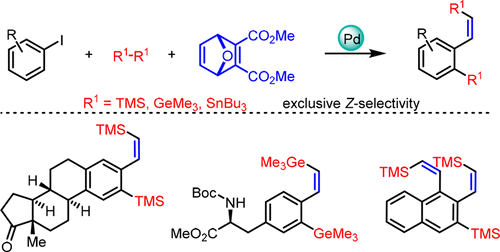
Palladium-Catalyzed Ortho-Silylation of Aryl Iodides with Concomitant Arylsilylation of Oxanorbornadiene: Accessing Functionalized (Z)-β-Substituted Vinylsilanes and Their Analogues - Org. Lett. - X-MOL
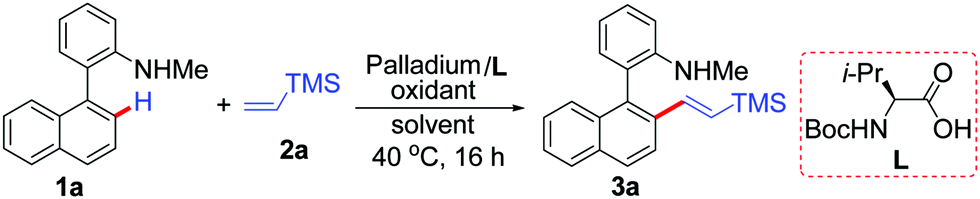
PdCl2(CH3CN)2-catalyzed regioselective C–H olefinations of 2-amino biaryls with vinylsilanes as unactivated alkenes - Chemical Communications (RSC Publishing)

Synthetic Applications of Allylsilanes and Vinylsilanes - Luh - - Major Reference Works - Wiley Online Library

Synthetic Applications of Allylsilanes and Vinylsilanes - Luh - - Major Reference Works - Wiley Online Library
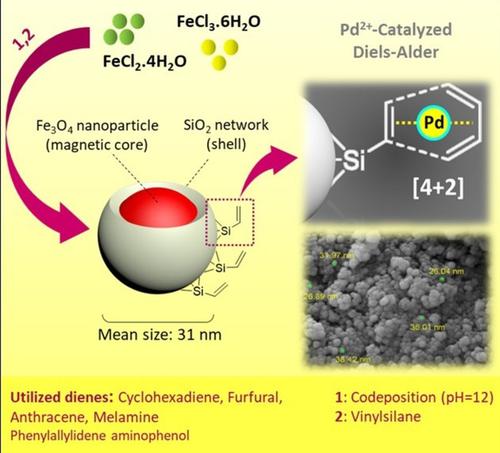
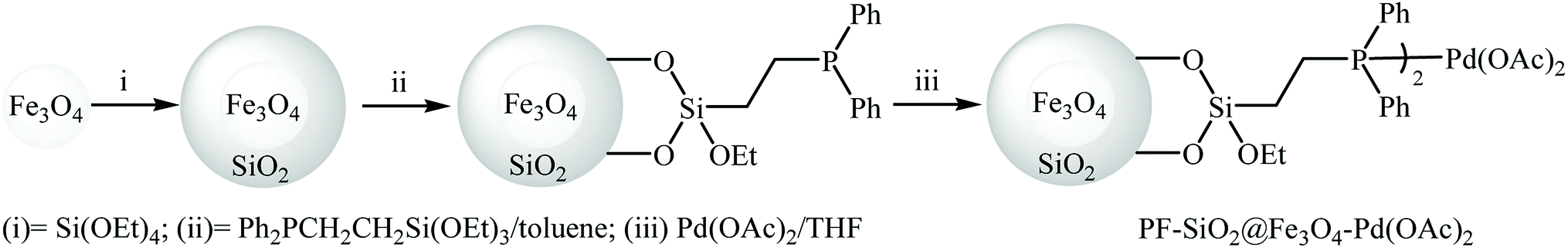
Surface functionalization of magnetic nanoparticles via palladium‐catalyzed Diels‐Alder approach - ChemistrySelect - X-MOL

Divergent Synthesis of Vinyl‐, Benzyl‐, and Borylsilanes: Aryl to Alkyl 1,5‐ Palladium Migration/Coupling Sequences - Han - 2020 - Angewandte Chemie - Wiley Online Library
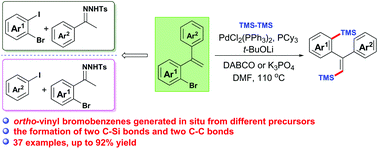
Pd-Catalyzed one-pot synthesis of vinylsilanes via a three-component tandem reaction - Organic Chemistry Frontiers (RSC Publishing)

Preparation of Allyl and Vinyl Silanes by the Palladium‐Catalyzed Silylation of Terminal Olefins: A Silyl‐Heck Reaction - McAtee - 2012 - Angewandte Chemie - Wiley Online Library

A Bithiophene‐Promoted ppm Levels of Palladium‐Catalyzed Regioselective Hydrosilylation of Terminal Allenes - Chen - 2020 - Advanced Synthesis & Catalysis - Wiley Online Library
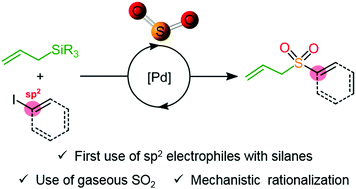
SO2 conversion to sulfones: development and mechanistic insights of a sulfonylative Hiyama cross-coupling - Chemical Communications (RSC Publishing)